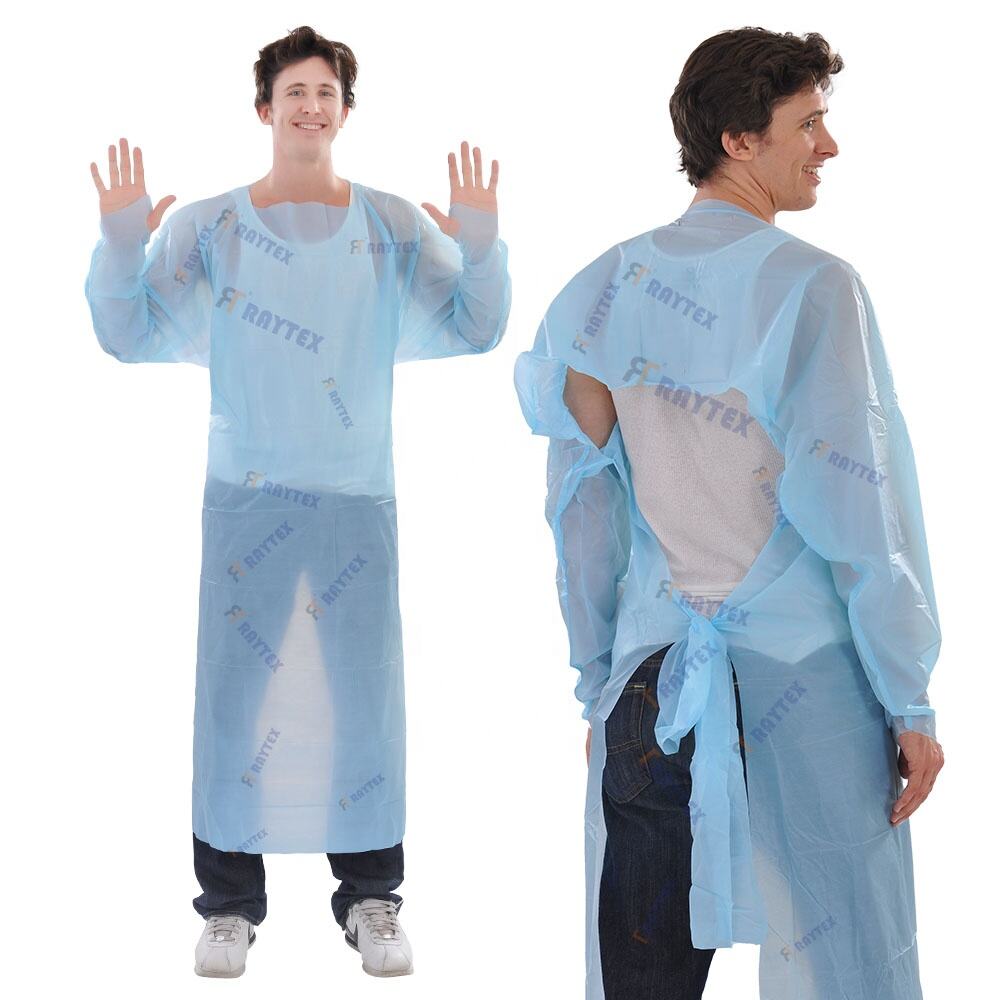
CPE (chlorinated polyethylene) gowns for pharmaceutical cleanrooms are specialized protective garments designed to maintain sterility and prevent contamination in controlled environments where pharmaceutical products, such as APIs (active pharmaceutical ingredients), biologics, and sterile formulations, are manufactured, processed, or packaged. These gowns are engineered to meet the stringent requirements of cleanroom classifications (ISO 5 to ISO 8), where even minute particles or microbial contaminants can compromise product integrity and patient safety. Constructed from high-quality CPE film, a durable, lightweight material known for its excellent barrier properties against liquids, particulate matter, and microbes, these gowns provide a reliable shield without shedding fibers—a critical feature in cleanrooms where particle counts are strictly regulated. CPE’s resistance to chemicals, including disinfectants and sanitizers used in cleanroom maintenance, ensures the gown remains intact during decontamination procedures, maintaining its protective efficacy over extended wear. The design of CPE gowns prioritizes full-body coverage and a secure fit, with features such as long sleeves with elastic cuffs, a high neckline, and a back or front closure (often using ties or hook-and-loop fasteners) to eliminate gaps where contaminants could enter. Many models are knee-length or longer, covering street clothing to prevent particle shedding from personal attire into the cleanroom environment. The gowns are typically disposable to avoid cross-contamination risks associated with reusable garments, which can harbor particles or microbes even after sterilization. Compliance with pharmaceutical standards is rigorous, with gowns meeting EN 13795 (surgical clothing and drapes) for barrier efficiency and ISO 14644-1 for cleanroom particle control. They are often sterile-packaged and gamma-irradiated to ensure they do not introduce microbial contaminants into the controlled environment. Additionally, CPE gowns are tested for static dissipation or anti-static properties in cleanrooms with sensitive equipment, preventing electrostatic discharge that could damage electronic components or disrupt manufacturing processes. By integrating these gowns into cleanroom protocols, pharmaceutical facilities adhere to Good Manufacturing Practices (GMP), minimize batch failures due to contamination, and ensure compliance with regulatory bodies such as the FDA and EMA, ultimately safeguarding patient health and maintaining the integrity of pharmaceutical supply chains.